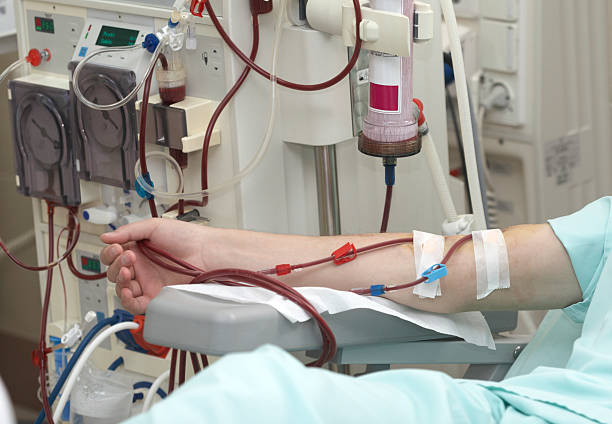
What is ECMO and Perfusion?
Extracorporeal Membrane Oxygenation (ECMO) is a critical care life support technique used for patients with severe heart and/or lung problems. ECMO can be employed in severe cases including cardiac or respiratory failure when conventional therapies have failed. The ECMO system works by temporarily taking over the function of the heart and lungs by oxygenating blood outside of the body. This process helps provide oxygen to the body’s tissues while allowing the heart and lungs to rest and heal. Perfusion, a fundamental component of ECMO and critical care medicine involves the circulation of oxygenated blood to the body’s tissues and organs. During the ECMO procedure, the perfusionist manages the entire operation of the extracorporeal circuit.
The Standard ECMO Circuit
An ECMO and perfusion circuit comprises several key components that facilitate extracorporeal support and adequate blood flow. These include cannulas, tubing, blood pumps, membrane oxygenators, heat exchangers, pressure monitors, and filters.
Cannulas and Tubing: Cannulas are flexible tubing that are inserted into large blood vessels. They withdraw blood from the body to the ECMO circuit and then return the oxygenated blood to the body. There are two main types of Cannulation, Vent-Arterial (VA) ECMO and Veno-Venous (VV) ECMO. In VA ECMO, blood is taken from a vein and returned to an artery, whereas VV ECMO blood is taken from and returned to a vein. These cannulas connect to larger tubing, which then circulates blood throughout the ECMO system.
Blood Pump: The tubing is connected to a blood pump which moves the blood through the circuit at a predetermined rate, ensuring adequate flow to the patient’s tissues. Depending on the ECMO system, the pump can be either centrifugal or roller type. Centrifugal pumps work by using a rotor known as an impeller to accelerate blood outward by centrifugal force and creating a pressure differential to propel the blood through the circuit. Roller pumps on the other hand work by compressing a segment of flexible tubing using rotating rollers. As the rollers move, they compress the tubing and move the blood forward in a peristaltic motion.
Membrane Oxygenator (artificial lung): The blood flows to the membrane oxygenator which is a critical component of the circuit to perform the gas exchange. This device works by using a gas permeable hollow fibre membrane to perform gas exchange, example in Figure 4. The gas exchange membrane consists of a large quantity of hollow fibres connected together. Gas exchange works as blood flows on the outer walls of the membrane while an oxygen rich sweep gas flows inside the fibres. Blood can be successfully oxygenated due to the gas permeable nature of such materials.
Heat Exchanger: After oxygenation, blood passes through a heat exchanger to ensure that the blood returns to the patient at an appropriate physiological temperature.
Pressure Monitors and Filters: Pressure monitors provide continuous blood pressure readings to ensure adequate blood flow. Filters remove air bubbles and debris from the blood before it re-enters the body, minimizing the risk of emboli and other complications.
Heat Exchanger: After oxygenation, blood passes through a heat exchanger to ensure that the blood returns to the patient at an appropriate physiological temperature.
Pressure Monitors and Filters: Pressure monitors provide continuous blood pressure readings to ensure adequate blood flow. Filters remove air bubbles and debris from the blood before it re-enters the body, minimizing the risk of emboli and other complications.
Common Materials used in ECMO and Perfusion

There is a wide range of materials used in the construction of an ECMO circuit. The majority of components are fabricated using polymers which include polyvinyl chloride (PVC), polyurethane, silicone, and polyethylene. These polymers are used due to their inherent flexibility, and high level of biocompatibility and they also have the ability to withstand blood flow without material degradation or harmful effects to the blood.
Metals such as stainless steel or titanium are also used within an ECMO circuit. They are used within the heat exchanger and blood pump. The material selection is chosen based on ample biocompatibility as well as their corrosion resistance properties.
As previously mentioned, the oxygenator membrane is crucial in the blood oxygenation process therefore material selection is of critical importance to achieve acceptable levels of gas exchange while preventing plasma leakage. There are two main materials used within the oxygenator, the first being polymethylpentene (PMP). PMP membranes are generally used in ECMO procedures where long-term device use (up to 30 days) is required. This is due to the material’s high efficiency in gas exchange and durability preventing plasma leakage. The other is polypropylene (PP) which similar to PMP has a high level of gas permeability but does allow plasma leakage. For this reason, PP is used in short-term (less than 6 hours) oxygenators for cardiopulmonary bypass procedures as PP due to its lower cost compared to PMP and is suitable for disposable applications.
Common Problems in ECMO Circuits and Why Hemocompatible Coatings play an Important Role
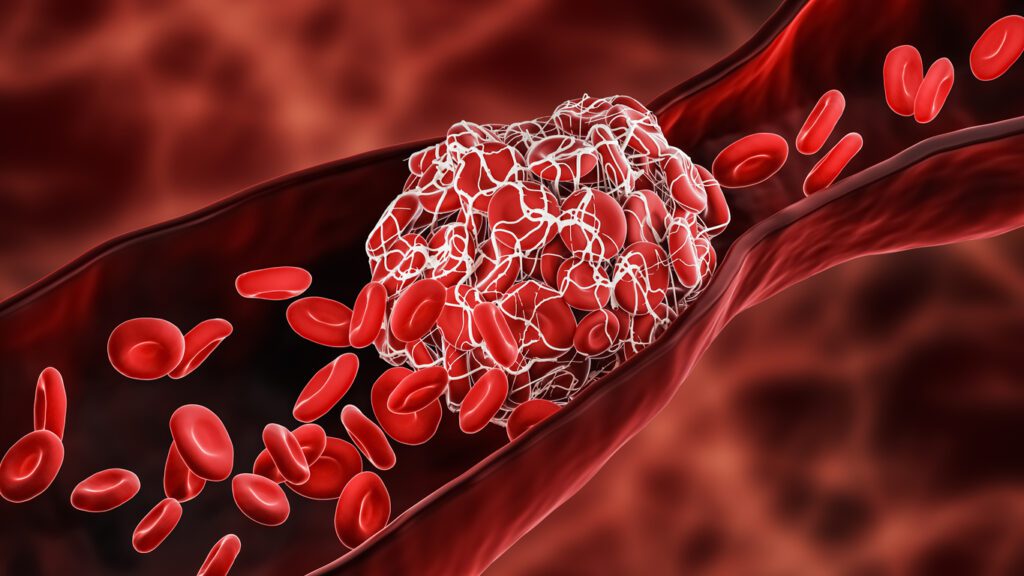
Despite their life-saving potential, ECMO and perfusion systems present challenges, including the risk of blood clot formation, infection, and mechanical complications. One of the main reasons for thrombus formation is the poor hemocompatibility characteristics of the materials used in ECMO circuits. Therefore, hemocompatible coatings are used to prevent the initiation of the clotting cascade and reduce the risk of thrombus formation
When evaluating the suitability of a hemocompatible coating for use in ECMO circuits, several critical factors must be considered. Firstly, it is essential to assess whether the hemocompatibility of the coated parts has improved. This involves testing the various components under dynamic blood flow and measuring key biomarkers that could indicate the initiation of blood clot formation. Secondly, the gas exchange capabilities of the oxygenator membrane must be evaluated to ensure that the coating does not impede device performance. This is achieved by comparing the oxygen and carbon dioxide levels on the venous and arterial sides of the oxygenator to determine the efficiency of oxygen and carbon dioxide transfer through the membrane and coating.
Smart Reactors Camouflage™ hemocompatible coating has been proven to enhance the hemocompatibility of ECMO and perfusion devices. This ultra-thin coating can be applied to all parts of the ECMO circuit, including the gas exchange membrane, without hindering device performance or preventing gas exchange within the oxygenator. Unlike competitor coatings, Smart Reactors proprietary coating, trademarked as Camouflage™ has been validated to ensure no blockage to the small channels between the individual gas fibres which can have a significant effect on device performance. Therefore, efficient gas exchange, increased hemocompatibility, and durability are the key performance characteristics of Smart Reactors CamouflageTM coating.
How to Evaluate a New Coating for ECMO and Perfusion Equipment
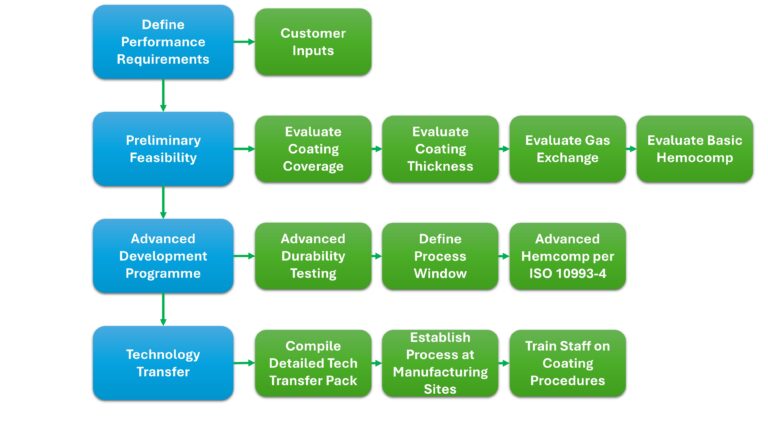
When deciding on a new hemocompatible coating for your device it is important to know the appropriate steps required to evaluate that coating and determine its suitability. The Figure below illustrates a summary of the process to be undertaken for qualifying Smart Reactors CamouflageTM coating on ECMO and Perfusion equipment.
Research and Development in ECMO and Artificial Lung Technology
There is currently a significant amount of ongoing research to improve ECMO performance technology, in particular, a targeted focus on developing new materials for the internal gas exchange membrane. Smart Reactors, in collaboration with leading experts from top universities, are investigating the fabrication of a novel nanocellulose gas exchange membrane for use in ECMO circuits. CellMembrane is a collaborative program funded by the European Union that aims to develop a nanocellulose membrane that is sustainable, affordable, biocompatible, and will mimic the gas exchange processes within the human lung. The output of this research program aims to revolutionize the design of artificial lung technology for longer-term use, bridging the gap to transplantation. For updates on the CellMembrane project follow the CellMembrane project’s LinkedIn and Twitter page.
Conclusion
ECMO and perfusion represent significant advancements in critical care, providing vital support to patients with severe heart and lung conditions. The ECMO circuit can temporarily take over the functions of the heart and lungs, offering a lifeline during critical periods. However, the use of ECMO is not without risk which includes the formation of blood clots. The application of hemocompatible coatings, such as Smart Reactors Camouflage™ coating is vital to reduce such clotting risks and enhance the longer-term hemocompatibility of ECMO/CPB devices. Ongoing research such as the work conducted in the CellMembrane project aims to tackle the hemocompatibility issues associated with the current state-of-the-art gas exchange membrane. This will be achieved by investigating new novel materials, including nanocellulose, which will improve the efficiency and longevity of ECMO circuits for better patient outcomes.
Share this post: on LinkedIn