What are Venous Stents And Flow Diverters?
Venous stents are medical devices that support weakened or narrowed blood vessels restoring blood flow. Initially developed to address coronary artery disease, stents can now be used for various applications including peripheral artery disease and cerebrovascular disease, and even have non-vascular applications. Stents are critical to the medical device industry as they restore blood flow to the heart or other affected areas. This helps prevent serious complications such as heart attacks and strokes, while keeping blood vessels open and ensuring that organs receive an adequate blood supply, thereby safeguarding against damage or dysfunction.
Flow diverters are specialized medical devices primarily used in the treatment of large or complex brain aneurysms. They function by redirecting blood flow away from the aneurysm, promoting circulation along the natural pathway of the blood vessel. This reduces the risk of aneurysm rupture which could lead to a haemorrhagic stroke. Flow diverters have a cylindrical mesh-like structure that is specifically designed to alter the flow of blood within the vessel.
This is achieved by placing the device at the neck of the aneurysm within the blocked vessel. The dense mesh structure of the flow diverter slows down the flow of blood to the aneurysm while maintaining circulation within the vessel. Reduced blood flow induces thrombus formation within the aneurysm effectively sealing it off. Over time the vessel wall begins to heal and strengthen. Flow diverters are crucial devices as they reduce, the need for open brain surgery thus providing a less invasive alternative
Complications and Clinical Challenges Associated with Stents and Flow Diverters.
Venous stents and flow diverters, while highly effective in managing vascular conditions such as chronic venous insufficiency and intracranial aneurysms, are not without their associated complications. A primary concern is the formation of thrombus (blood clots), which can precipitate severe events like stroke, deep vein thrombosis (DVT), potentially life-threatening pulmonary embolism (PE) if a clot dislodges and travels to the lungs. To mitigate this risk, dual antiplatelet therapy is often employed to prevent clot formation; however, this comes at the cost of an increased risk of bleeding. Blood filters present a potential adjunctive solution by capturing clots before they can cause harm, thereby providing an additional layer of protection. In the context of flow diverters, delayed endothelialization or incomplete aneurysm occlusion can compromise device efficacy, leading to potential failure. Furthermore, restenosis remains a significant concern, often driven by factors such as vessel obstruction, external compression, or suboptimal stent design, all of which can impair long-term outcomes.
Procedure For Stent And Flow Diverter Placement
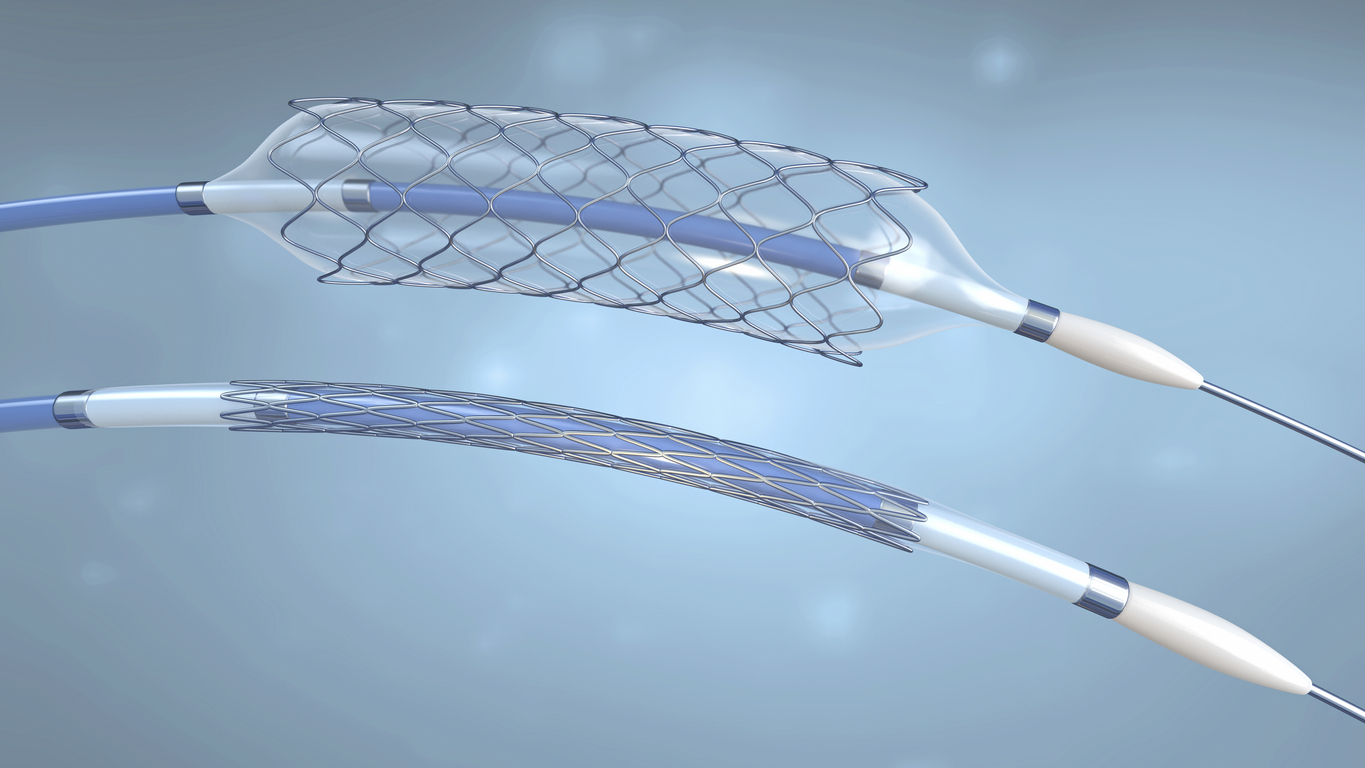
The procedure involved in placing a stent or flow diverter is minimally invasive compared to surgical alternatives. In preparation, the patient receives a general or local anesthetic depending on the type of procedure being carried out. A thin, flexible tube known as a catheter is placed into a large artery in the groin or arm to allow for the stent or flow diverter to be inserted.
Using fluoroscopy, the device is then guided to the desired location. Prior to this procedure, the stent is crimped to reduce the size of the device to allow ease of access through the patient’s vascular system. When the stent is positioned in the desired location, a small ballon is used to expand the stent allowing it to support and open the affected vessels. Alternatively, a self-expanding stent can be used which doesn’t require a balloon catheter.
Post-procedure patients may need to remain in the hospital for observation, though the duration of stay is typically much shorter compared to more invasive surgeries. In many cases, patients utilize a combination of dual antiplatelet therapy and blood filters, working synergistically to effectively prevent thrombus formation. Concerns regarding patient adherence to such medication are the primary driving forces for the use of hemocompatible coating technologies on such devices to reduce the risk of thrombus formation.
Common Materials Used In Stent & Flow Diverter Manufacturing
Stents and flow diverters can be made from a variety of materials including metal alloys, polymers, or a combination of materials. Generally, nitinol and cobalt chromium are the primary materials used when fabricating stents and flow diverters.
Nitinol is a metal alloy composed of equal parts nickel and titanium. This material is used in the field of medical devices due to its super elastic properties making it up to 10 times more flexible than other metals as well as its thermal shape memory properties. This shape memory effect describes a metal that can undergo a reversible phase transformation between Austenite and Martensite. Under high temperatures, nitinol enters the Austenite phase. In this phase, it achieves maximum stiffness and is spring-like when bent. During low temperatures, nitinol enters the Martensite phase. In this phase, the metal feels rubbery and bends easily.
In its Martensite state, nitinol can be easily set into a new shape. However, when heated through its transformation temperature, it reverts to Austenite and returns to its previous shape.
Slight changes in the alloy composition or heat treatment affect the temperature at which nitinol remembers its high-temperature form.
The specific application for the device determines the optimal transition temperature. In the case of stents, a transition temperature close to or equal to human body temperature is selected allowing the stent to revert to its original shape post crimping.
Cobalt chromium is another commonly used material for stents but is mainly used in flow diverter manufacturing. This is due to its high corrosion resistance and biocompatibility which reduces complications with surrounding tissue when implanted. Additionally, it is a chemically inert material that can minimize the possibility of irritation, allergic reaction, and immune response. Cobalt chromium is also an extremely strong alloy allowing for thinner struts while maintaining radial strength.
Selection of Stent Materials: The Importance of Hemocompatible Coatings
There are a variety of risks associated with stents and flow diverters including stent restenosis, which is the narrowing of the stent area after treatment (in arterial vessels), and the risk of blood clot formation. While the materials used for stents and flow diverters have a natural level of biocompatibility, it is insufficient to prevent thrombus formation and thus risking health complications for the patient. It is recommended that blood thinning medication is taken to minimize the initiation of the blood clotting cascade.
Restenosis is the most common clinical complication associated with arterial stents. Drug-eluting coatings are used to reduce potential restenosis. However, venous stents or neurovascular flow diverters carry a higher risk of thrombosis.
Drug-eluting stents help to prevent restenosis by using a thin coating that delivers medication directly to the bloodstream and the arterial walls. This inhibits cell proliferation to mitigate cell growth through the spaces between struts. Consequently, this disrupts endothelization of the stent surface and re-endothelization of the vessel walls. The resulting consequence is the delay in the healing process of the vessel wall as well as preventing long-term hemocompatibility and the risk of thrombus formation further down the line. There are also a variety of complications associated with long-term exposure to the medications used in drug-eluting stents. Drug-eluting coatings are not considered a desirable solution in the venous or neurovascular system as restenosis is not the major cause of clinical failure. Drug-eluting coatings gradually deplete their drug supply over time, which can lead to the re-emergence of complications as the coating loses its therapeutic effect.
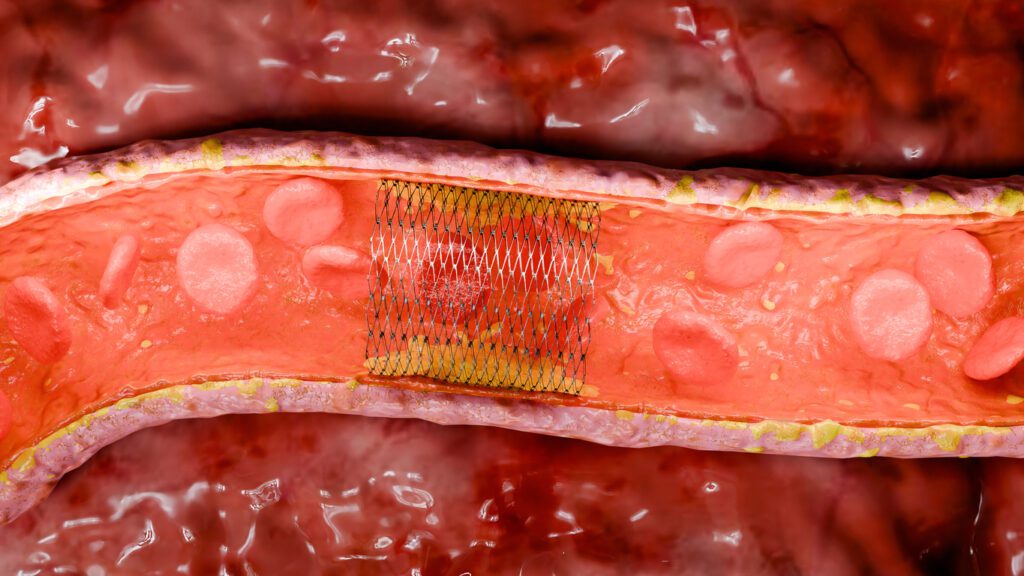
A more favorable method for the prevention of thrombus formation is through the use of hemocompatible coatings such as Smart Reactors Camouflage™ Coating Technology.
There are many factors to be considered when selecting an appropriate hemocompatible coating for use with stents & flow diverters. Firstly, the coating must improve the hemocompatibility of the device surface preventing the coagulation cascade. The coating must also be highly durable both to blood flow and high mechanical force. As mentioned above, a stent undergoes crimping for deployment, so it is important that the coating is not damaged by this process. Finally, the coating must promote endothelization on the surface of the stent or flow diverter. This will allow for long-term hemocompatibility significantly reducing the risk
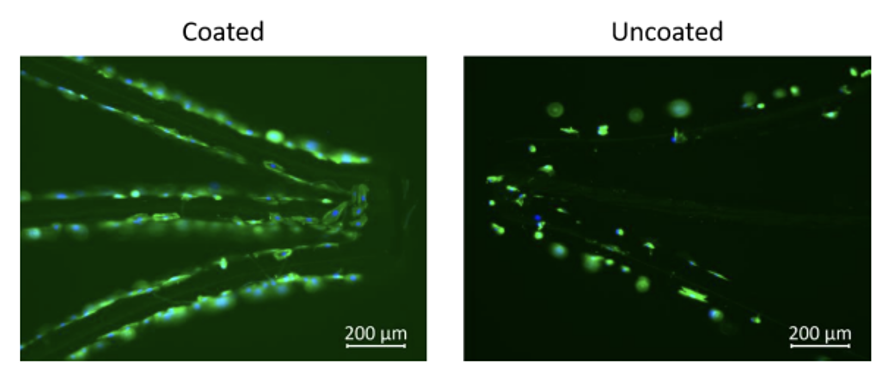
of thrombus formation. All of this can be achieved with Smart Reactors Camouflage™ which demonstrates excellent hemocompatibility, long term durability and promotes the endothelization process. The Figure above illustrates the growth of endothelial cells on the surface of a Camouflage™ coated stent when compared to an uncoated control. Additional benefits of Smart Reactors Camouflage™ coating include its ability to exhibit all the desired characteristics outlined above, without the use of pharmaceuticals. As a result, patients are not exposed to long-term medication, unlike with drug-eluting stents.
Methods For Testing Hemocompatible Coatings For Stents And Flow Diverters
When determining an appropriate hemocompatible coating for a stent or flow diverter, it is important to understand the methods used to evaluate the coating. Firstly, it is vital to ascertain whether the applied coating improves the hemocompatibility of the device surface. There are a variety of methods used, however, the most widely used is the Chandler Loop system.
This functions by placing a coated stent or flow diverter inside a closed loop of PVC tubing filled with human blood. This is then rotated to allow the blood to interact with the device surface while in flow. Post-testing, the blood is analyzed for biomarkers that indicate the activation of the coagulation cascade. Based on the detected biomarkers, the level of hemocompatibility can be determined. Smart Reactors Camouflage™ coating has been demonstrated to improve the hemocompatibility of stents and flow diverters through Chandler Loop testing.
Methods For Evaluating Endothelisation Properties On Stents And Flow Diverters
It is crucial that the coating on the surface of a stent or flow diverter promotes endothelization. This can be determined through static cell culturing techniques. In this method, the coated stent or flow diverter is placed in a cell culture medium alongside Human Umbilical Vein Endothelial Cells (HUVECs) and left for a period of time ranging from 1 to 14 days. Once the culture period has been reached, cell adhesion to the device surface can be determined through fluorescent microscopy or scanning electron microscopy.
Following on from this, the cytotoxic effects of the coating are evaluated. This verifies that the cells which have grown on the surface of the coated stent or flow diverter are alive and healthy. This is achieved using cytotoxicity assays and cell counting. Smart Reactors Camouflage™ has been demonstrated to be non-cytotoxic to endothelial cells.
Conclusion
Stents and Flow Diverters are crucial in the medical device industry providing lifesaving treatment with minimally invasive procedures. Stents help support weakened or blocked blood vessels while flow diverters are crucial in the management of complex brain aneurysms. However, with all blood-contacting medical devices, there is a risk of thrombus formation due to poor material hemocompatibility. This highlights the vital importance of hemocompatible coatings such as Smart Reactors Camouflage™ to help reduce the risk of blood clot formation improving the long-term hemocompatibility of Stents and Flow Diverters.
Current Publications
Share this post: on LinkedIn