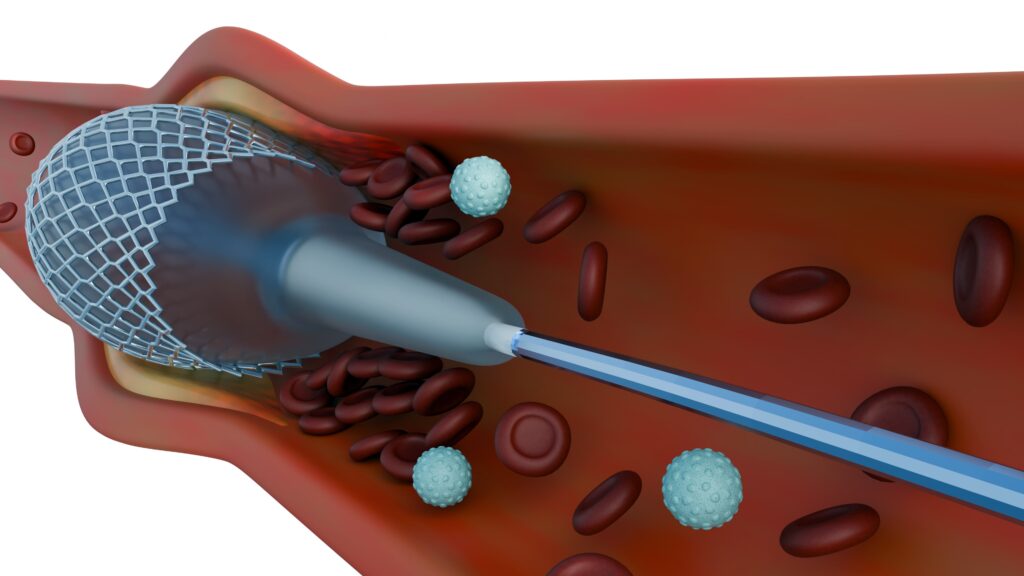
The heart relies on the seamless separation of blood flow. When congenital defects like Atrial Septal Defects (ASDs) and Patent Foramen Ovale (PFOs) create abnormal openings in the septal walls, they disrupt circulation and increase the risk of stroke and heart failure.
Septal occluders are innovative devices that non-invasively close these defects, restoring proper blood flow. Unlike traditional open-heart surgery, these nitinol-based implants are deployed via a catheter-based procedure, minimizing recovery time. Over time, the device integrates into the heart’s natural structure.
However, biological responses to foreign materials can impact the success of these devices. Without the right modifications, complications like thrombosis, inflammation, and delayed endothelialization can arise—potentially leading to serious risks for patients.
The Challenges with Septal Occluders
Although septal occluders provide a less invasive alternative to open-heart surgery, their introduction into the bloodstream triggers a biological response. Without the right modifications, these devices can lead to complications such as:

- Thrombogenicity: Blood clot formation on the device surface can obstruct normal blood flow, increasing the risk of embolism or stroke.
- Delayed Endothelialization: The body’s ability to cover the implant with natural tissue is crucial for long-term success. Without sufficient endothelialization, patients remain at risk for thrombosis and require prolonged anticoagulation therapy.
- Foreign Body Response: The immune system may recognize the device as a foreign object, causing inflammation, excessive fibrotic tissue formation, or even device rejection.
This is where advanced coatings, like Camouflage™ becomes essential.
The Critical Role of Coatings in Septal Occluders
1. Preventing Thrombosis: Blood-contacting surfaces risk platelet adhesion and fibrin buildup, which can lead to clot formation. Camouflage™ coating significantly reduces thrombogenicity, ensuring safer interaction with blood flow.
2. Promoting Rapid Endothelialization: Successful integration depends on tissue growth over the implant to form a natural barrier. Camouflage™ promotes endothelial cell adhesion, reducing reliance on anticoagulants and accelerating healing.
3. Reducing Inflammatory Responses: Foreign body reactions can cause chronic inflammation and fibrosis, threatening device longevity. Camouflage™ is engineered to minimize immune system activation, ensuring long-term stability.
What Sets Camouflage™ Apart?
Smart Reactors has engineered Camouflage™ to address the specific challenges of blood-contacting implants. This proprietary water-based, non-heparin coating offers:
1. Biocompatibility – Prevents thrombus formation while maintaining blood compatibility.
2. Superior Durability – Adheres to all medical-grade materials without requiring UV or thermal crosslinking.
3. Regulatory Advantage – Designed for seamless approval pathways with medical regulatory bodies.
As the need for advanced septal occluders continues to grow, Camouflage™ technology is redefining safety and performance in cardiovascular implants.
Partner with Smart Reactors Today
At Smart Reactors, we specialize in next-generation biocompatible coatings that enhance cardiovascular device performance. Whether you’re developing septal occluders, vascular implants, or blood-contacting devices, Camouflage™ is the key to unlocking superior patient outcomes.
Contact Smart Reactors today to learn how Camouflage™ can elevate your device performance.
Share this post: on LinkedIn